Digitalisation, modernisation, and evolving patient expectations are pushing the pharmaceutical industry towards new horizons. While these are a few factors that affect all industry players, one key focus is cost effectiveness. This can relate to the ingredients being used, or the formulation process itself. Tackling this issue can be extremely valuable for formulators, whilst additionally having a direct positive effect on patients/end users.
IMCD’s technical experts are patient-centric in their approach. IMCD has numerous case studies and innovations that present cost-effective solutions from its global network of technical centres.
One such example of a sustainable, cost-effective solution comes from IMCD's Mumbai Pharmaceutical Technical Centre (PTC). In terms of decreasing manufacturing stages, inventory, cleanliness, production time, and energy consumption, direct compression (DC) is a considerably faster and economical technique compared to dry granulation (DG). However, the DC technique is the least advantageous when dealing with moisture-sensitive actives (APIs) that usually have poor flow and compaction.
The scientists of IMCD’s PTC Mumbai recognised the issues that their global customers faced when dealing with Sitagliptin, one of the most well-known anti-diabetic drugs. Remarkably, they were able to develop and test a robust, cost-efficient, and sustainable formulation for both slugging and roller-compaction processes. The high level of moisture sensitivity prevents one from using a MADG (moisture-activated dry granulation) process.
Table 1: Post-formulation characteristics of IMCD Sitagliptin formula
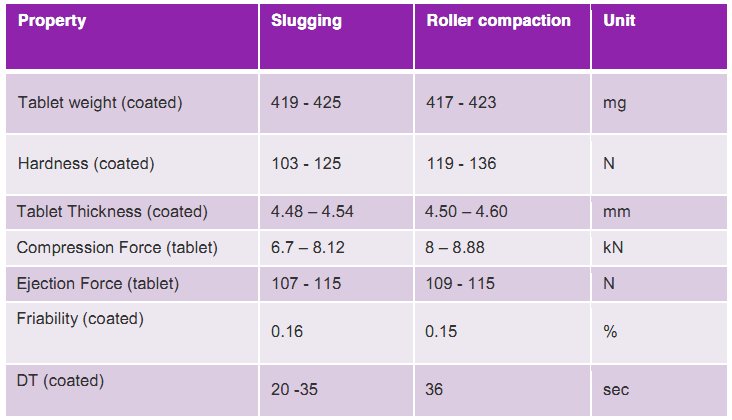
The alternative was to opt for a special grade of anhydrous dicalcium phosphate, which when placed in a formulation, can be used in different manufacturing processes. The team evaluated the potential of using this grade of DCPA for moisture-sensitive APIs using both roller compaction and slugging methods. Additionally, a special coating grade was used with a PVA-PEG graft polymer that is favourable when working with moisture-sensitive drugs. This instant release coating is highly impermeable to water – making it ideal for moisture-sensitive active ingredients and significantly increasing formulation stability. IMCD developed a cost-effective formulation using the sustainable process of dry granulation.
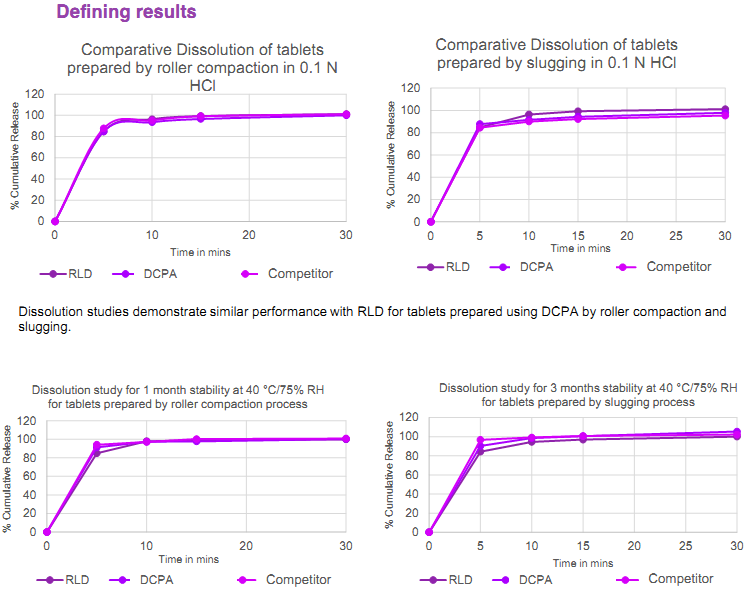
The post-formulation results show that IMCD’s formula is equally robust for roller compaction and slugging methods. Additionally, the coating is ideal for any moisture-sensitive drug and better in many ways for a standard coating process. This highly flexible film former can be prepared at room temperature, and no plasticiser is required. Additionally, this polymer has low viscosity, which allows for the creation of spray suspensions with a solids content of 20-25%, resulting in exceptionally cost-efficient manufacturing processes. Furthermore, IMCD’s three-month stability data showed excellent results and the dissolution profiles from the trials matched those of the originator. Indirectly, IMCD demonstrated formulation and process feasibility that can be beneficial for other moisture-sensitive APIs like Amlodipine Besylate, Sildenafil Citrate, Rosuvastatin, Varenicline Tartrate, Isosorbide Dinitrate, Efavirenz and Chlorzoxazone.
Fig. 1: Results of dissolution tests following roller compaction and slugging in different media
IMCD has developed a reliable formulation at its Mumbai Pharmaceutical Technical Centre that is effective, long lasting, stable, and reasonably priced. It's crucial to provide patients with diabetes access to high-quality, reasonably priced medications because the disease is so persistent and chronic and requires daily treatment. IMCD is pleased to collaborate with and influence pharmaceutical companies to provide them with highly effective yet cost-effective patient solutions.

