Bilayer tablets are an under-utilized option that can be employed to help reduce treatment burden, but their formulation is more complex than for conventional monolayer products.
The oral administration of drugs has persistently been the preferred route of drug delivery for many years, mainly due to patient convenience and manufacturability of the dosage form. “Tablets are the most stable and used oral sold dosage (OSD) form on the market that allows for drug price reductions while also guaranteeing good production yields combined with patient compliance,” asserts Federica Giatti, compression technologist at IMA Group.
However, conventional immediate-release tablets are designed to release an active ingredient shortly after oral administration, which generally means a rapid absorption of drug occurs without any specific targeting of therapeutic action. Various methods have been employed to be able to manipulate the release of APIs in the body, in order to control the therapeutic efficacy of treatments, improve patient compliance, and reduce dose frequency.

Bilayer tablets have been employed as a means of administering more than one API in a single dosage orm to achieve controlled drug delivery. Although these dosage forms can offer key advantages, there are also specific complications and challenges that must be overcome to ensure success.
Benefits of bilayer tablets
In the modern era, continuous scientific research into disease areas and multi-targeted approaches is opening a wide range of possibilities in pharmaceutical development and manufacturing, Giatti confirms.
“The possibility to use bilayer tablets offers some advantages that must be taken into consideration when developing a combination of two or more APIs in a single pharmaceutical form,” she says. Some advantages of bilayer tablets include the option of fine-tuning the availability of each API with the possibility of differentiating their release-times, being able to separate incompatible APIs, prolonging the medicine effect after administration, and reducing the dose frequency, Giatti highlights.
For example, she continues, in elderly patients, the high number of tablets or medicines that may be required each day can impact quality of life or can lead to medication errors, so bilayer tablets can be particularly beneficial for this patient population.
“Bilayer or multi-layer can provide the delivery of more than one drug in one dose thereby adding convenience,” concurs Anthony Carpanzano, director of R&D at JRS Pharma. “Delivery in separate layers can avoid incompatibilities between the compounds that otherwise could not be prevented in a single monolithic structure. Multiple layers also allow for delivery of the components at different rates and visually, multi-layer tablets provide brand recognition.”

Formulation considerations
Formulating a bilayer tablet is more complex than for a conventional monolayer tablet as two (or more) suitable granulations must be developed. “Generally, to have a blend suitable for compression, the powder should be free-flowing and uniform in terms of particle size distribution; it should be characterized by particles cohesive enough to lock and hold together but not too much so they can adhere to metal surfaces,” notes Giatti.
“Blend characteristics, such as compactability, flow, homogeneity, and low dustiness – to minimize ‘cross-contamination’ between layers – are important,” agrees Carpanzano. “Flow is especially important for layers two and beyond.”
By guaranteeing the blend characteristics, the final tablet should be uniform and defect-free, Giatti continues. “Compatibility is another crucial characteristic to be considered. If the APIs inserted in the formulation are incompatible, they must be separated in different layers and formulated with dedicated release,” she says. “Last but not least, the sufficient bonding between the two layers must be guaranteed even immediately after tableting and during storage, packaging, and shipping.”
To ensure a stable tablet process, when developing and manufacturing a bilayer tablet, manufacturers must choose the correct tamping and pre- and main-compression forces, and formulation scientists need to select an excipient that is suitable for direct compression, which will allow for easy flowability, compression, and tableting of the blend, Giatti explains. “The tableting should be performed without any contamination between the two layers,” she adds. “[Formulators can improve the chances of being contamination-free] by choosing powder with a coarser particle size that can reduce blend volatility around the machine, and manufacturers assist with dedicated aspiration.”
Most of the challenges to overcome when developing a bilayer tablet pertain to process or equipment issues, emphasizes Carpanzano, but other significant considerations include layer adhesion and layer thickness versus tooling shape – in particular, cut depth.
A greater amount of attention should be focused on the bonding between the two layers, asserts Giatti.
“From a manufacturer point of view, this [bonding issue] can be solved by applying a slight tamping force in the first layer, whereas for formulators the inner features of all the excipient and APIs present (crystalline habitus, real density, particle size distribution) should be taken into consideration from the beginning,” she says. But, for Giatti, the most significant challenge to overcome is in relation to ncompatibilities of the ingredients. “Different release profiles and physical separation of the APIs should be ensured by choosing the relevant type and percentage of excipient(s) or manufacturing technology to use,” she states.
Additionally, the development of technologies designed for the compression of multilayer tablets has matured with systems now at the point of simply needing ‘fine-tuning’, reveals Carpanzano. “More development of compression coating or ‘tablet-in-tablet’ is ongoing,” he adds.
Software is available that can help developers in choosing the correct formulation, such as IMAGO
(IMA Group), highlights Giatti. “This software consists of an instrument that acquires the data of the cells mounted in the machine, such as pre-main compression and ejection force, as well as tablet characteristics (weight, thickness, diameter, and tablet strength) to consider powder behavior,” she explains. Other software exists that guide the choice of ratio and type of excipient, such as ZoomLab by BASF, Giatti continues. “The combination between these aids and the experience of the supplier and manufacturer, can improve and facilitate the choices of the formulators,” she says.
Excipient selection
“Excipient selection for bilayer tablets is as important as it is for single-component tablets, and there are instances when it is most important,” Gernot Warnke, global head of R&D at JRS Pharma stresses. “For example, when running a high-speed tablet press, the filling of the die with the blend to be compressed occurs when the die passes below the feed frame and the lower punch is drawn downward. This downward motion happens quickly and creates a degree of suction which aids in filling the die. This suction helps to compensate for materials with less than optimal flow in achieving uniform die filling and ultimately consistent tablet weight uniformity.”
However, Warnke warns, for multi-layer tablets, the benefits of the downward motion of the punch are only achieved for the first or lower layer of the tablet. “Any subsequent layers must rely solely on blend flow to fill the die cavity. It is therefore imperative to select excipients that impart the best flow characteristics to provide adequate flow characteristics to subsequent layers,” he says.
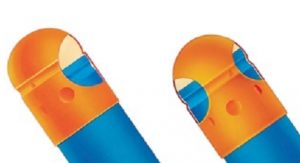
Selecting the optimal excipient for the first layer is also important to ensure the surface, after tamping, has a rough enough porosity to allow for adequate adhesion of the second layer, continues Warnke. “It has been shown that if the first layer is too smooth (due to makeup or excessive tamping) the second or subsequent layer will not adhere well and layers may separate with minimal agitation (i.e., during ejection, discharge, packaging, or over the shelflife of the product),” he says.
“Due to the fact that the first layer [in a multi-layer tablet] is generally subjected to low compression forces, the formulation should be able to cope with further compression in the second phases of tableting without leading to a fragile tablet,” adds Giatti. Furthermore, selecting an excipient with good compressibility can improve the overall tableting output and help to overcome the potential problem of later separation and delamination and capping, she notes.
“To conclude,” continues Giatti, “the flowability of the formulation of the first and second layer should be good enough to not generate any issues during the feeding of the tablet press that could cause a reduction of output or [overall equipment effectiveness] of the manufacturing equipment.”
Regulatory factors
Widely encompassing regulatory factors are to be considered when approaching bilayer tablets: following the correct good manufacturing practice approach to ensure a high-quality tablet is produced that complies with the standard customer specifications and pharmacopeial requirements, specifies Giatti. “At the same time, tablets must be defect-free, capping and separation of the two individual layers should be prevented, and the final tablet hardness has to be sufficient to cope with downstream processes or handling,” she adds.
 A deep analysis of any incompatibility between the API and excipient is critical, and the cross contamination among the layers should be avoided, asserts Giatti.
A deep analysis of any incompatibility between the API and excipient is critical, and the cross contamination among the layers should be avoided, asserts Giatti.
“Finally, the two layers should be clearly separated to ensure the correct API release, to avoid undesired contamination, and for aesthetic reasons,” she says.
“Bilayer (and multi-layer) products fall into the category of combination products, and thus must have a therapeutic ‘reason for being’,” states Carpanzano. “Clinical studies to support the combination must therefore be established and approved.” The analysis, method development, validation, and quality control of bilayer tablets is more complex than for monolayer products and, as a result, can be a more time-consuming process, he notes.
An under-used option
Ongoing research into various disease areas that demand a multi-targeted approach is broadening the potential utility of bilayer tablets for developers, asserts Giatti. “[Bilayer tablets offer developers] a real chance to reduce the number of medicines being taken by a patient, increasing medication compliance on one hand, and reducing the potential for administration error on the other, which in turn increases the therapeutic effect of medicines being prescribed,” she says. “In my opinion, multi-layer tablets are still under-utilized and can offer cost-effective alternatives to much more complex drug delivery options, both in immediate- release as well as in modified-release formats,” summarizes Warnke. “Even with the advent of 3D printing of tablets, multi-layer tableting remains a much cheaper option, especially in the case of high production volumes.
Contact Information:
Mr. Zoran Bubalo:
tel.: +38 063 442 5648
Zoran@bubalo.rs