Introduction
Introduction
The enteric coating was developed in the 1940s to act as a barrier for drug protection with low acidic stability from degradation by gastric acid or to protect gastric mucosa from irritating drugs. Moreover, it allows to deliver drugs optimally absorbed in the small intestine in a most concentrated form in this specific region of the digestive tract.
Based on hypromellose, acetyl and succinoyl groups are introduced to the hydroxyl groups of the backbone and different pH openings are obtained by variating their amount.[1] Hypromellose acetate succinate (HPMCAS) was developed by Shin-Etsu in 1980s as an enteric coating polymer under the brand name Shin-Etsu AQOAT® and firstly approved in Japan in 1987.[2]
HPMCAS is commonly used in oral pharmaceutical formulations as a film coating, as well as an enteric coating material for tablets or granules. It acts as a solubility enhancing agent via amorphous solid dispersion. Hypromellose acetate succinate is insoluble in gastric fluid, but will swell and dissolve rapidly in the upper intestine. [3]
Talc is widely used in oral solid dosage formulations as a glidant, lubricant and diluent, as a dissolution retardant in the development of controlled-release products or as an adsorbant.[4] It is also commonly used as antiadhesive in enteric coating formulations including the different dissolution method for Shin-Etsu AQOAT®.
In this particular study we will investigate the impact of Talc in enteric coating formulations using Shin-Etsu AQOAT® as enteric polymer in aqueous coating dispersion.
The amount of Talc will be continuously decreased using two different grades of Talc, which are commonly used in the pharmaceutical industry with different particle sizes – Talc MS (D50~ 11µm) and Micro Ace P3 (micronized grade D50~5 µm) from Nippon Talc (Japan).
We assume that proper enteric formulations are possible using lower amount of Talc without losing any properties of the coating and avoiding nozzle clogging, especially with a bigger particle size grades of Talc in the formulation. Moreover, we assume using micronized grade will improve the formulation and show stable results compared to bigger particle size grades.
Materials and Methods:
Materials:
The materials used in this study are listed below: Aqoat AS-MF (Shin Etsu Chemical, Tokyo, Japan), Talc Micro ACE P3 (Nippon Talc, Osaka, Japan), Talc MS (Nippon Talc, Osaka, Japan), Sodium Dodecyl Sulfate (Merck, Darmstadt, Germany), Ammonia 20,5% (Avantor, Gliwice, Poland), Citrofol AI – TEC (Jungbunzlauer, Basel, Switzerland), HarkePlus Tab (Harke Pharma, Muelheim a. d. R., Germany), Ligamed MF-3-V Magnesium stearate (Peter Greven, Bad Munstereifel, Germany), purified water.
Equipment and conditions:
The equipment and tableting conditions were set as follows: Erweka RTP D8 rotary tablet press (Erweka, Langen, Germany), Ø 9 mm round, spherical r = 11 punches; Drum Coater Farma-Tech D-300 (Farma-Tech, Kutno, Poland); IKA RW 20.n (Ika Labortechnik, Staufen, Germany), equipped with stainless steel propeller stirrer; Phenom Pro Scanning Electron Microscope (Phenom World, Eindhoven, Netherlands) - detection: BSD, 10-15 kV accelerating voltage, Keyence VHX-7000 digital microscope (Keyence, Osaka, Japan), Testo 830-T4 Infrared thermometer (Testo, Pruszków, Poland). Conditions: Precompression: 350 N, Main compression: 15 kN, Rotor speed: 40 rpm. Composition of placebo tablets: 99.5% HarkePlus Tab, 0.5% Magnesium Stearate.
Preparation of coating mixtures and coating conditions:
The coating compositions were partially neutralized, where 20% of acid groups are neutralized. This method allows partial polymer dissolution and higher HPMCAS load.
All the mixtures were prepared according to the following procedure: i) Amount of needed water was added into beaker, ii) SLS was added to the stirred water, stirred for 5 min until dissolved, avoiding formation of foam, iii) Aqoat AS-MF was added and stirred fast for 10 min, iv) Ammonia solution was added and stirred for 5 min at normal speed (Ammonia amount is necessary calculated from succinoyl content of HPMCAS), v) Required talc grade was added and stirred fast for 10 min (note: no “ultra turrax” used for better visualization of differences between the talc grades), vi) TEC was added and stirred for 5 min at normal speed.
The coating conditions were set as follows: Drum Coater Farma-Tech D-300 (Farma-Tech, Kutno, Poland) was used at a drum speed of 7 rpm, inlet air temperature of 75-85 °C, bed temperature of 35-40 °C, Nozzle Ø 0.8 mm, atomizing air: 2.0-2.1 bar, pump speed: 0.8 (according to device‘s scale) with a target mass increase of 10% (based on mass loss of coating mixture).
Eight batches in total were prepared: Batch 1-4 using Talc Micro Ace P3 grade with decreased amount going from 3.0%, 2.25%, 1.5% down to 0.75%; Batch 5-8 using Talc MS grade using the decreased amount of Talc of the same percentage.
Results and Discussion:
 In general no sticking of tablets was observed, regardless the coating composition. However, an excessive nozzle clogging was noticed in the coating process of batch 8 (Talc MS, 0.75%).
In general no sticking of tablets was observed, regardless the coating composition. However, an excessive nozzle clogging was noticed in the coating process of batch 8 (Talc MS, 0.75%).
Besides clogging, the tablets showed a tendency to detach the applied functional coat, especially on the edges for the same batch. Therefore, coating of this batch can be defined as a failure.
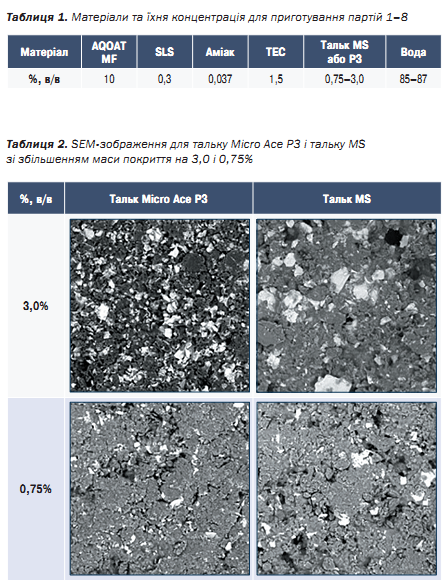
After the mixture was prepared and the tablets were coated, the surface of the coating were evaluated with SEM. For the reasons of the better overview in this publication, we show only the results from 3% and 0.75% for both Talc grades.
As expected, the SEM pictures of the both grades of Talc showed a little more homogenic surface for the lower amount of Talc, since this mineral is only dispersible, but not soluble in water.
However, it is also expected from the coating that the dissolution profile should work well and should not deviate from the expected profile.
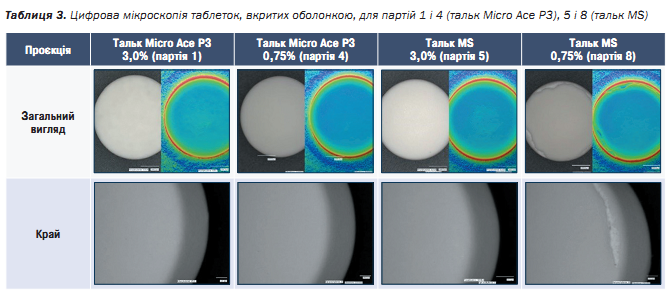
Furthermore, the tablets were investigated under the digital microscope from different points of view. In order to maintain clarity, we put the focus only on the overview of the tablet and their edges, investigating Batches 1 (3%) and 4 (0.75%) of Micro Ace P3 Talc and Batches 5 (3%) and 8 (0.75%) of Talc MS. The results are shown in the table below.
The investigation of the tablets with digital microscopy showed, that using 3% of each Talc grade have led to a successful coating with no holes, nozzle clogging during the process or broken edges.
However, decreasing the Talc amount in the coating formulation for Micro Ace P3 Talc showed still a very impressive results, wherein doing the same for Talc MS led to a poor result. The coating of batch 8 was detached to the edges of the round tablets, which can be seen in the Table 3 above. Moreover nozzle clogging was observed during the process, which might have happened due to a poor dispersion of the coating mixture itself.
Conclusion:
All the batches 1-4 coated with compositions containing Micro Ace P3 have been coated successfully, with no issues encountered during coating process. The quality of the coat, as assessed with microscopic observations, is good, and no differences have been noted regarding the used content of the talc.
The batches 5-8 coated with compositions containing Talc MS have been coated successfully, when Talc content was 3.0%, 2.25%, and 1.5%. However, when Talc MS content was decreased down to 1.5% in the tablet crossection, “layering” of tablet coat was observed, which was not the case in the composition containing Talc P3 (not shown in this publication).
When the Talc content was decreased to 0.75%, the coating was unsuccessful due to nozzle clogging and poor coat adhesion to the tablet cores, especially on the tablet edges (Table 3).
No visible differences between tablets coated with Micro Ace P3 and Talc MS have been observed with talc concentration down to 1.5%. Since no sticking of the tablets was noticed, it can be assumed that the decreased amount of Talc, even of different grades, show similar results and does not affect the coating properties itself.
However Micro ACE P-3 performed better at lower concentrations, which might be caused by a much smaller particle size and a bigger surface area of the material. This means for a successful coating less amount of Talc is needed, which can save and improve preparation efforts and time, costs and processing of the coating solution itself.
Further investigation process will focus on mechanical properties and solubility of coating films, especially when the Talc content is low. The dissolution profile using an API will be compared with the used Talc grades and the results evaluated compared to a standard formulation.
References:
[1] Shin-Etsu AQOAT® product page: https://www.setylose.com/en/products/healthcare/shinetsu-aqoat (accessed 28.04.2023).
[2] Shin-Esu AQOAT® brochure, 2021/11.
[3] Handbook of Pharmaceutical Excipients, Hypromellose Acetate Succinate, p. 330, 6th Edition, 2009.
[4] Handbook of Pharmaceutical Excipients, Talc, p. 728, 6th Edition, 2009.