Introduction
Known for their convenience, tablets are the most popular oral solid dosage form preferred by both patients and manufacturers alike. Because tablets can be easily processed, carried, and dosed, this dosage form boasts high patience compliance among other desirable attributes. When manufacturing tablets, three main processing methodologies are commonly utilized: direct compression (DC), dry granulation, or wet granulation. DC is the preferred production method as it eliminates the need for additional processing steps required in dry and wet granulation, e.g., ribbon milling and binder addition, respectively, ultimately allowing for a reduction in manufacturing time and costs.
However, DC formulations typically also require multiple excipients to fulfill flowability, compressibility, disintegration, and lubrication requirements. Coprocessed excipients, which combine two or more functional materials into a single ingredient, can be used to overcome these formulation challenges through their enhancement of the resulting material’s performance and processability. By reducing manufacturing complexity and thus drug development time, coprocessed excipients can significantly expedite time-to-market.
Case study
Vardenafil HCl is a phosphodiesterase-5 inhibitor indicated to treat erectile dysfunction. It is slightly soluble in water, so it tends to be micronized into a fine, white powder with a wide particle size distribution (Table 1). Vardenafil micronized particles have an irregular shape that negatively impacts drug processability and compressibility (Figure 2). A direct compression (DC) process of this active pharmaceutical ingredient (API) is not easily accomplished through traditional methods without pre-processing via wet granulation, for example, to enhance flowability. These formulation challenges can be resolved employing a highly flowable, coprocessed excipient designed for direct compression processes.
 Kollitab™ DC 87 L is an all-in-one coprocessed excipient developed for DC of solid oral dosage forms. It was strategically designed to encompass all basic excipient functionalities required for tablet manufacturing: lactose monohydrate as the filler, fine crospovidone (Kollidon® CL-F) as the disintegrant, Kollicoat® IR as the binder, and sodium stearyl fumarate (SSF) as lubricant. This study aimed to produce vardenafil HCl and Kollitab™ DC 87 L tablets by direct compression.
Kollitab™ DC 87 L is an all-in-one coprocessed excipient developed for DC of solid oral dosage forms. It was strategically designed to encompass all basic excipient functionalities required for tablet manufacturing: lactose monohydrate as the filler, fine crospovidone (Kollidon® CL-F) as the disintegrant, Kollicoat® IR as the binder, and sodium stearyl fumarate (SSF) as lubricant. This study aimed to produce vardenafil HCl and Kollitab™ DC 87 L tablets by direct compression.
KollitabTM DC 87 L was strategically designed to encompass all basic excipient functionalities required for tablet manufacturing:
- Lactose monohydrate as the filler
- Fine crospovidone (Kollidon® CL-F) as the disintegrant
- Kollicoat® IR as the binder
- Sodium stearyl fumarate (SSF) as lubricant
Materials and methods
Vardenafil HCl and Kollitab™ DC 87 L were characterized by particle size distribution (PSD), flow, compression, and disintegration time. First, vardenafil HCl and Kollitab™ DC 87 L were accurately weighed and dispensed. Then, to produce 1 kg of the 2 % vardenafil blend, vardenafil was passed through a 0.6 mm sieve and blended with Kollitab™ DC 87 L in a lab-scale V-blender for 15 minutes at 17 rpm.
Results and discussion
Kollitab™ DC 87 L is a free-flowing powder (Figure 1) produced via a spray-drying process. This coprocessed excipient has a median particle size of ~160 µm (Table 1) and has a spherical morphology as shown in Figure 3. In contrast, vardenafil HCl is an off-white, micronized, and cohesive powder (Figure 2) that has a high span value and is characterized by non-uniform shard-like particles (Figure 4).
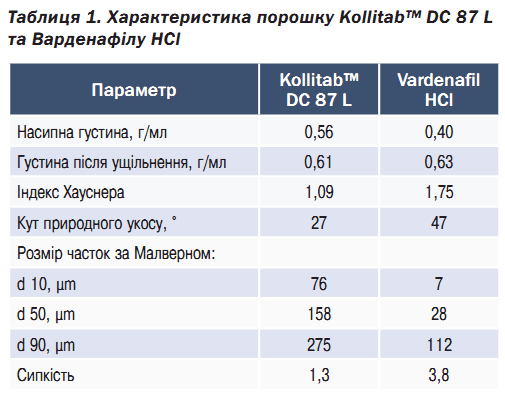
Governed by its spherical particles, Kollitab™ DC 87 L has excellent flowability, evidenced by the low angle of repose and Hausner ratio (Table 1). In comparison, vardenafil HCl has poor flowability, leading to a need for extra attention during the formulation development process as this undesirable attribute risks poor content uniformity and high tablet weight variability during the tableting process.
The FT4 Powder Rheometer was used to perform compressibility and shear cell tests for Kollitab™ DC 87 L, vardenafil, and a 2 % drug load mixture. Compressibility (%) analysis was used to measure the percentage of change in volume as a function of applied force.
Materials that show high compressibility are likely to be cohesive, compact more, and have poorer flow. Shear cell data was important for understanding how the powder flows from hoppers and funnels, and its propensity to form bridges or rat holes during compres sion or other dynamic processes. A Flow Function coefficient (FFc) was calculated from the shear cell data results. A higher FFc correlates to better flow due to lower internal forces (Van der Waals, etc.)
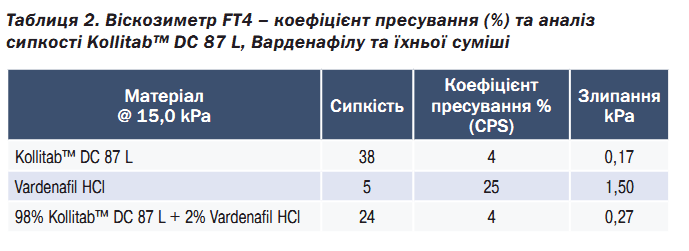
As shown in Table 2, Kollitab™ DC 87 L showed low compressibility (%), low cohesion, and a high flow function. Based on these results, Kollitab™ DC 87 L was shown to not have a tendency towards agglomeration; rather, this coprocessed excipient remained free flowing regardless of the handling conditions (storage, filling/dispensing). In contrast, vardenafil HCl had the lowest FFc and highest compressibility and cohesion which is typical of materials that agglomerate and flow poorly. When both materials were blended at 2 % drug load, the addition of Kollitab™ DC 87 L improved vardenafil flowability, reducing cohesiveness to improve drug compressibility and processability via direct compression.
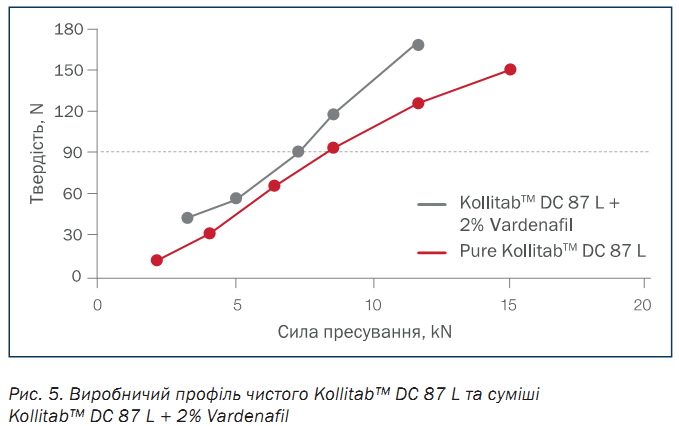
Kollitab™ DC 87 L and the blend of Kollitab™ DC 87 L + 2 % vardenafil HCl were directly compressed into 250 mg tablets using 9.0 mm round punches with a KORSCH XL 100 tablet press. A range of compression forces were evaluated to define the manufacturability profile of pure Kollitab™ DC 87 L and the 2 % drug blend, as presented in Figure 5.
With an ideal tablet hardness considered to be ten times (10x) that of the tablet size, the desired hardness of the 9.00 mm tablets was 90 N. Figure 3 shows that pure Kollitab™ DC 87 L required a compression force of 9.0 kN to form a tablet with the target hardness of 90 N. When Kollitab™ DC 87 L was blended with 2 % vardenafil, a lower compression force of 7.0 kN was required to obtain the same hardness of 90 N. While vardenafil’s irregularly shaped micronized particles can negatively impact drug compressibility, the incorporation of Kollitab™ DC 87 L in the 2% API formulation allowed for the use of minimal compression force to manufacture the tablet via DC and achieve the ideal tablet hardness of 90 N. In summary, Kollitab™ DC 87 L provided high tablet strength at low compression forces which allowed for manufacturing benefits including the improvement of tableting process robustness and reduction of machine and punch damages caused by excessive compression force.
As a part of the drug development process, formulators and scientists need to carefully select materials that have excellent compressibility, but minimal impact on tablet disintegration and drug dissolution. While some cellulose excipients prolong disintegration time, particularly when using high compression forces, Kollitab™ DC 87 L offers a fast disintegration time for low and high-strength tablets due to its unique composition which includes fine crospovidone
(Kollidon® CL-F). This superdisintegrant instantly swells upon contact with water to explode the tablet. Due to its particle size, it also works as a binder to aid during compression. Kollitab™ DC 87 L’s composition also contains SSF, a less hydrophobic lubricant with almost no impact from overblending.
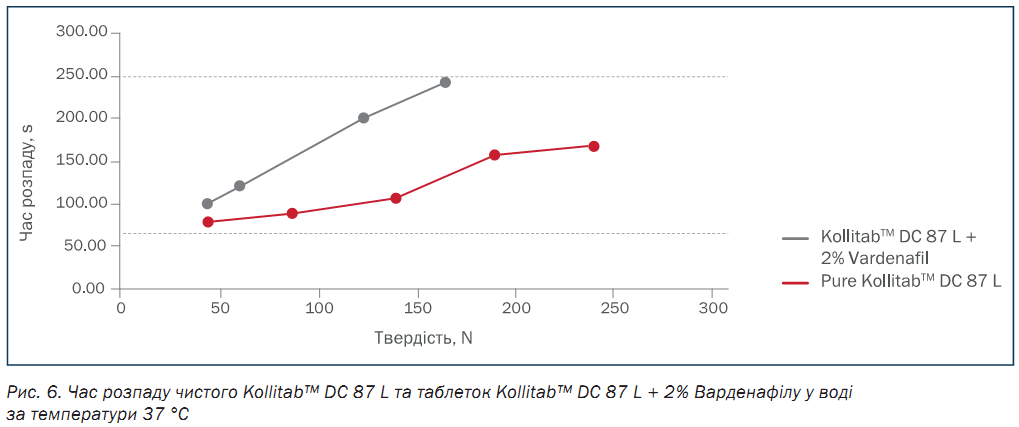
Figure 6 shows the disintegration time of tablets made with pure Kollitab™ DC 87 L and the Kollitab™ DC 87 L + 2 % vardenafil blend across a broad hardness range. All tablets disintegrated within 5 minutes, regardless of their hardness.
Conclusion
 Direct compression is the most common method to produce tablets; however, DC can sometimes require specialized excipients to achieve an efficient and robust process. All-in-one coprocessed excipients are multifunctional materials strategically designed with the formulator in mind. These are manufactured by combining key excip ients into one material to achieve optimal blend processability and product performance. Kollitab™ DC 87 L is the newest all-in-one coprocessed excipient, containing all the functionalities required to produce tablets: lactose monohydrate as the diluent/filler, crospovidone as the disintegrant, Kollicoat® IR as the binder, and sodium stearyl fumarate (SSF) as lubricant.
Direct compression is the most common method to produce tablets; however, DC can sometimes require specialized excipients to achieve an efficient and robust process. All-in-one coprocessed excipients are multifunctional materials strategically designed with the formulator in mind. These are manufactured by combining key excip ients into one material to achieve optimal blend processability and product performance. Kollitab™ DC 87 L is the newest all-in-one coprocessed excipient, containing all the functionalities required to produce tablets: lactose monohydrate as the diluent/filler, crospovidone as the disintegrant, Kollicoat® IR as the binder, and sodium stearyl fumarate (SSF) as lubricant.
Kollitab™ DC 87 L is the ideal solution to overcome direct compression challenges posed by cohesive APIs such as vardenafil HCl. Its use can provide high process consistency and reduced variability, in addition to fast disintegrating tablets at all compression forces, with no ejection problems.
In conclusion, blending Kollitab™ DC 87 L together with APIs such as vardenafil HCl promotes superior flowability, high tablet strength at a broad range of compression forces, and fast disintegration time. These advantages can result in simplifying the drug development process, reducing manufacturing costs, and expediting time-to-market.
TC AURORA LLC
62 Dehtyarivska Street,
Kyiv 04112,
Ukraine
Tel: +38 044 594 8777
sales@tc-aurora.com
www.aurora-pharma.com


